
Mendeleev and was later revised by Henry G. The periodic table was invented by Dmitri I. Atomic number of elements steadily increases across a period.
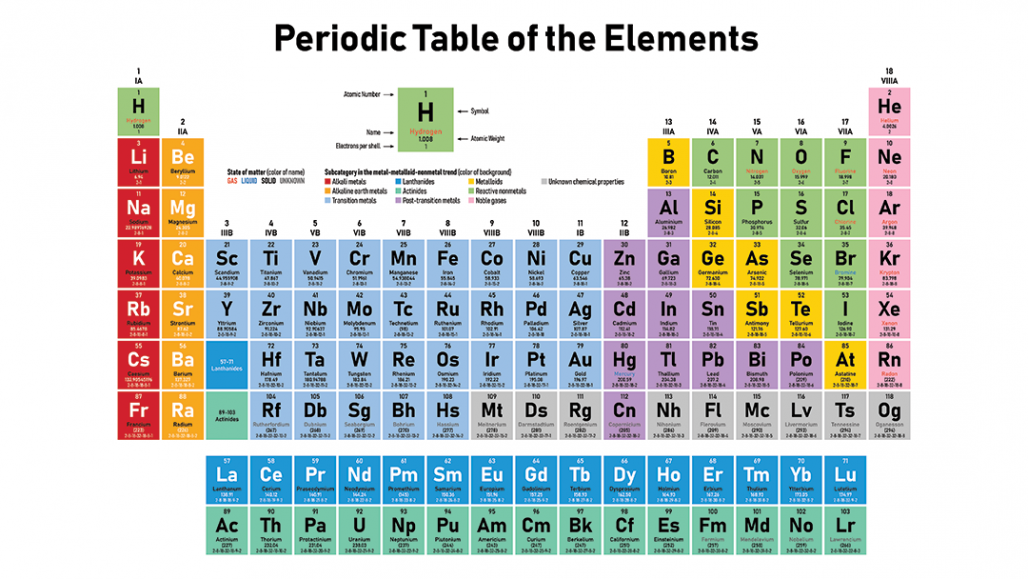
Also, figure out how and why elements interact to make the world you see around you. The periodic table is an arrangement of all the elements known to man in accordance with their increasing atomic number and recurring chemical properties. Since 1899 the IUPAC Commission on Isotopic Abundances and Atomic Weights ( CIAAW) has been evaluating atomic weights and abundances. The truth is that atomic weights have changed as a function of time. Comics include Uncle crooge, Metal Men, Metamorpho, Batman. Atomic weights found within a periodic table one might think are constant. For example, group 18 (or 8a) is the inert gases, which do barely interact chemically with other elements because they have no valence electrons.Each of the elements in a period (a row) have the same number of electron shells the number of electrons in these shells (the element's atomic number) increases from left to right. Elements in the periodic table are arranged by increasing atomic number (number of protons). Go through the Periodic Table piece by piece and learn how it works. This site contains comic book images linked to the chemical elements via the periodic table. In it, the elements are arranged by increasing atomic number (the number of protons in the nucleus of an atom).Įach of the elements in a group (a column) have qualities in common. The periodic table of the elements shows the types of elements that make up the universe and the relative properties of the atoms. Ideal if youre a student, teacher or just have an interest in the chemical sciences. Our subscribers' grade-level estimate for this page: This fact-filled, image-rich app is the only periodic table you need. Today's featured page: Kangaroo Theme Page The modern periodic table is based on Dmitri Mendeleevs 1896 observations that chemical elements can be grouped according to chemical properties they. The copy-paste of the page "Periodic Table Cipher" or any of its results, is allowed as long as you cite dCode!Ĭite as source (bibliography): Periodic Table Cipher on is a user-supported site.Īs a bonus, site members have access to a banner-ad-free version of the site, with print-friendly pages.Ĭhemical Elements Wheel - Common Metals - Bottom: Printable WorksheetĬhemical Elements Wheel - Common Metals - Top: Printable Worksheet
#ATOMIC TABLE ANDROID#
Except explicit open source licence (indicated Creative Commons / free), the "Periodic Table Cipher" algorithm, the applet or snippet (converter, solver, encryption / decryption, encoding / decoding, ciphering / deciphering, translator), or the "Periodic Table Cipher" functions (calculate, convert, solve, decrypt / encrypt, decipher / cipher, decode / encode, translate) written in any informatic language (Python, Java, PHP, C#, Javascript, Matlab, etc.) and all data download, script, or API access for "Periodic Table Cipher" are not public, same for offline use on PC, mobile, tablet, iPhone or Android app! Ask a new question Source codeĭCode retains ownership of the "Periodic Table Cipher" source code.
The concepts of nucleus (nuclear), atomic bomb, elementary particles (proton, electrons, neutrons), chemistry, etc.

The painting is associated with its author: Dmitry Ivanovich Mendeleev (sometimes written Dimitri) a Russian chemist. The periodic table is a table of the elements in order of atomic number, arranged in rows and columns to illustrate periodic similarities and trends in. Elements that have similar chemical properties are. A higher atomic weight than the one on its left. Mendeleev found he could arrange the 65 elements then known in a grid or table so that each element had: 1.

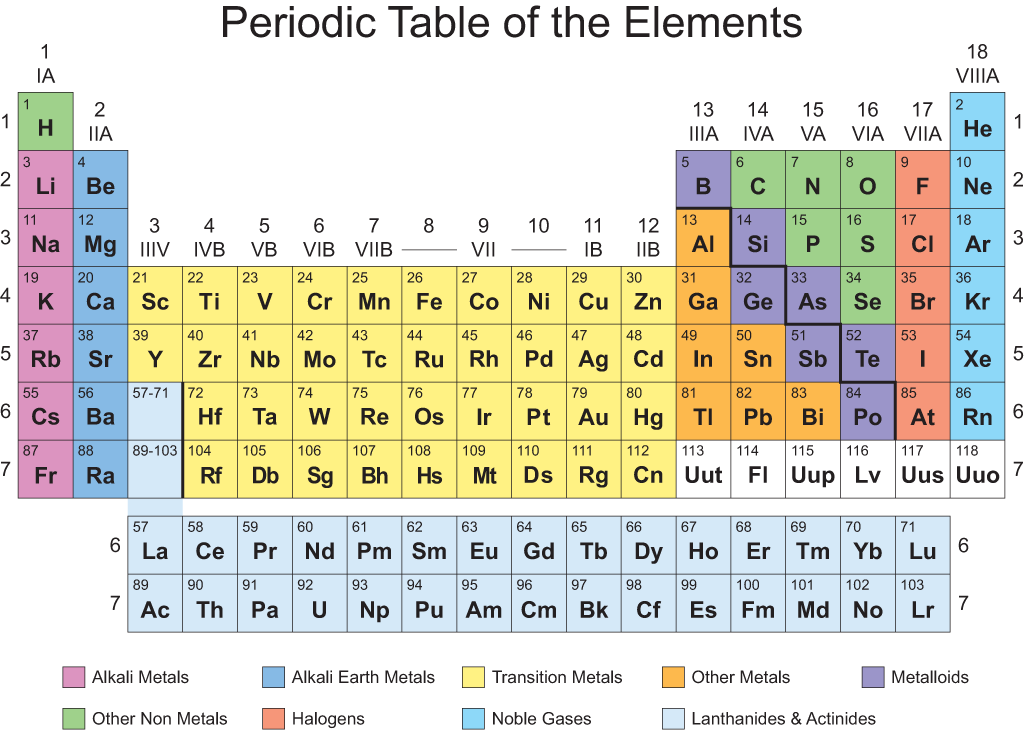
There you can find the metals, semi-conductor (s), non-metal (s), inert noble gas (ses), Halogens, Lanthanoides. The periodic table we use today is based on the one devised and published by Dmitri Mendeleev in 1869. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. The total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol Z. Please note that the elements do not show their natural relation towards each other as in the Periodic system. In the periodic table, the elements are listed in order of increasing atomic number Z. The unity for atomic mass is gram per mol. The message is made up of numbers between 1 and 118. A modern periodic table lists elements left to right by atomic number. The lightest chemical element is Hydrogen and the heaviest is Hassium.


 0 kommentar(er)
0 kommentar(er)
